Toward Membrane-Permeable Macrocyclic Peptides
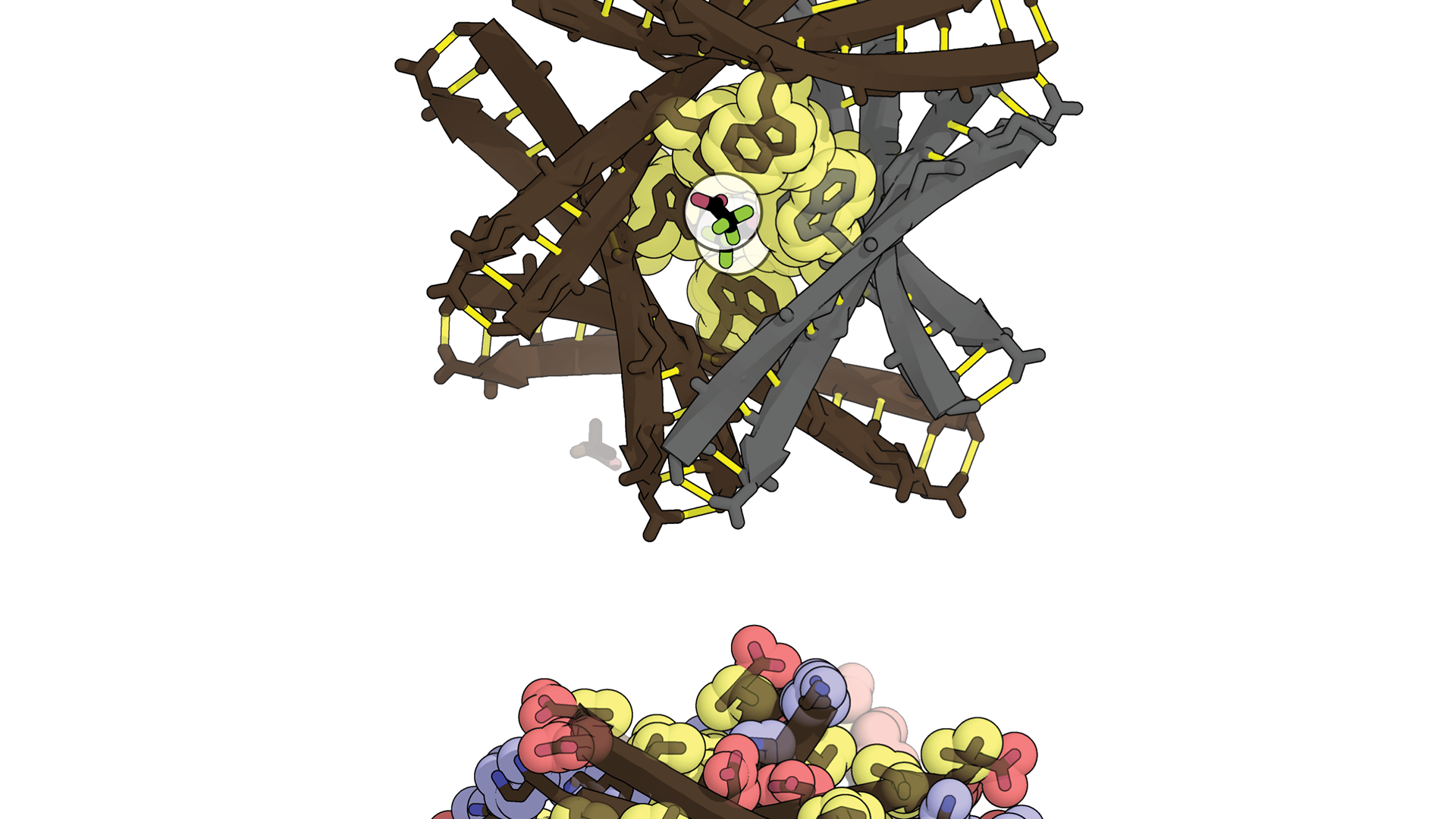
N-methylated macrocyclic peptides (N-MeMPs) are a promising class of cyclic compounds with medicinal potential, offering innovative applications in cargo delivery, tissue labeling, cellular imaging, and therapeutics. Unlike traditional small molecules constrained by Lipinski’s rule of 5, N-MeMPs, with molecular weights exceeding 600 Da, occupy a unique chemical space between small molecules and linear peptides, making them ideal candidates for targeting intracellular protein-protein interfaces (PPIs), 75% of which are considered “undruggable” by small molecules. Cyclosporine A (CycA), an 11-residue cyclic peptide that binds cyclophilin A and calcineurin, exemplifies the therapeutic potential of N-MeMPs. However, most successful N-MeMP drugs, including CycA, originate from natural products, and the rational design of membrane-permeable cyclic peptides remains a significant challenge. Membrane permeability is essential for targeting intracellular proteins, yet only 18 cyclic peptides have been approved for clinical use over two decades, with just two (Romidepsin and Voclosporin) targeting intracellular proteins—starkly contrasting the approval rates of small-molecule drugs. Addressing this bottleneck requires a comprehensive understanding of the factors governing N-MeMP membrane permeability, which is key to unlocking their pharmaceutical potential.
Fibril Formation and Crystallization of Metabolites
Recent discoveries have revealed that metabolites—including amino acids (e.g., Phe, Tyr, Met, Cys, Trp, Ile), secondary metabolites (e.g., orotic acid, oxalate), lipids (e.g., glucosylceramide), and nucleobases (e.g., uracil, adenine)—can form amyloid-like nanostructures, challenging the long-standing belief that only peptides and proteins aggregate into amyloid fibrils. These metabolite assemblies exhibit physicochemical properties akin to protein amyloids, such as shared morphology, dye-binding characteristics, electron diffraction patterns, immunological responses, and cytotoxicity. While most metabolite functions are attributed to their monomeric forms, little is known about their assembled states. This research aims to address this knowledge gap by investigating (1) the early stages of metabolite assembly and the factors governing transitions between fibril and crystal states, (2) the catalytic, templating, and seeding activities of metabolite fibrils, and (3) the structural and functional impacts of accumulated metabolites on cytotoxic, water-soluble peptide assemblies. Together, these studies will provide fundamental insights into the roles of metabolite assemblies in health and disease.
Duplicated Insulin Genes and Diabetes
This project investigates two murine insulin isoforms, Ins1 and Ins2, to uncover how structural differences drive functional diversity and influence diabetes risk and treatment outcomes. While humans have a single insulin gene, mice and rats possess two, a result of gene specialization through environmental adaptation. Amino acid differences between Ins1 and Ins2 are known to affect proteolytic processing, insulin aggregation, and responses to physical and chemical stress, as well as modulate the onset of insulin action and local insulin concentration and potentially introduce novel functions absent in monomeric insulin. However, the mechanisms by which insulin isoforms, mutations, post-translational modifications (PTMs), and aggregation contribute to diabetes onset and progression remain poorly understood. This project aims to address these knowledge gaps, providing insights that could inform the development of improved insulin therapies.